iHEAR® Medical, first to create FDA-cleared home hearing assessment kit, a breakthrough for home diagnostics
The FDA-cleared iHEARtest™ gives consumers control of their hearing health in the convenience and privacy of their own home, with affordable and innovative technology.
(San Leandro, CA) The availability of, and demand for, at-home diagnostic products is on the rise – the market for point-of-care diagnostics is projected to reach $37 billion by the end of 2021. Consumers are seeking options to assess their own health through affordable, technologically advanced yet simple to use self-monitoring solutions. The first Food and Drug Administration-cleared home hearing assessment kit, called the iHEARtest™, has been introduced by iHEAR® Medical to give consumers control of their own hearing health.
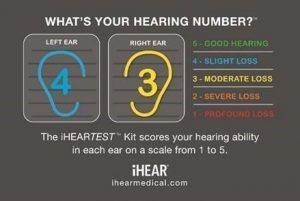
The Center for Hearing and Communication reports that 48 million Americans have a significant hearing loss, yet only 16% of physicians routinely screen for hearing loss. For the first time, consumers now have FDA-cleared technology allowing them to screen and monitor their own hearing from the convenience and privacy of their home. Consumers can take the 6 minute iHEARtest™ assessment and immediately learn what their Hearing Number™ is on a scale from 1 to 5 (from profound hearing loss to good hearing in each ear).
Hearing loss is on the rise. From 2000 to 2015, the number of Americans with hearing loss has doubled, according to the Hearing Health Foundation. Hearing loss may result from genetic causes, complications at birth, certain infectious diseases, chronic ear infections, exposure to excessive noise, and aging. The American Speech-Language Hearing Association recommends adults have their hearing checked at least once every 10 years up to the age of 50, then every three years.
“Millions of Americans suffer from a reduced quality of life due to their hearing loss. Our vision was to develop technology that empowers and informs consumers who want to monitor their own well-being. People with hearing loss can definitely benefit from early identification,” said John Luna, CEO of iHEAR® Medical, Inc. “The future of screening diagnostics is being driven by consumers who want to take many routine assessments in the privacy and convenience of their home. With the iHEARtest we’re making it possible for more people to incorporate hearing health into their wellness routine.”
iHEAR® Medical has made the iHEARtest™ accessible and available over-the-counter at independently owned pharmacies, national drugstore chains, and now online (including CVS.com, FSAStore.com and Walmart.com.) The iHEARtest™ kit includes a proprietary USB device that connects to a personal computer and factory-calibrated earphones. The user downloads the screening software and follows the instructions to administer the iHEARtest™. Using secure online access to a HIPAA-compliant server, the user can review their results at any time.
For more information on iHEAR® Medical, visit: www.ihearmedical.com
About iHEAR® Medical
What’s your hearing number?™ The future of better hearing technology has arrived with iHEAR® Medical. Dedicated to affordable and accessible hearing solutions, featuring state-of-the-art technology with an intuitive and elegant design, iHEAR® Medical has a list of firsts. Founded in 2010, iHEAR® Medical continues to shape the future of hearing solutions with the first FDA-cleared over-the-counter iHEARtest™ home screener, and its hearing aid solutions, iHEARhd®, iHEARmax™, Eva™ by iHEAR®, and the personal sound amplification product TReOTM by iHEAR® 3-in-1 Amplifier. iHEAR® holds more than 50 U.S. and international patents for its products and services.